
Next-Generation Skin Repair Module: From PDRN × Exosome to Peptides × Epi-On® × Azeclair® P Precision Regeneration and Homeostasis Strategy
Next-Generation Skin Repair Module: From PDRN × Exosome to Peptides × Epi-On® × Azeclair® P Precision Regeneration and Homeostasis Strategy
Corum Inc.
As Skin Repair Enters the Era of “Intelligent Regeneration,” the Focus Shifts from Single Ingredients to Precision Signaling and Microenvironment Rebalancing

In the new era of intelligent skin regeneration, the focus is no longer merely stimulating the skin with a single active ingredient. Instead, the core lies in precise signal modulation combined with microenvironmental homeostasis.
In recent years, PDRN (Polydeoxyribonucleotide) has been widely used in the fields of skin repair and regeneration. It has become a popular ingredient in both aesthetic medical treatments and skincare products. Its main mechanism involves the release of adenosine through metabolic breakdown, which activates A2A receptors and indirectly modulates anti-inflammatory and tissue repair responses, laying a solid foundation for skin regeneration.
However, as our understanding of epigenetic regulation and the skin’s intrinsic self-healing mechanisms has deepened, the limitations of this metabolite-dependent, indirect activation pathway have become increasingly evident:
Today, we are witnessing a shift from metabolic-dependent actives to a new generation of direct-acting molecules—led by innovations like Epi-On®.
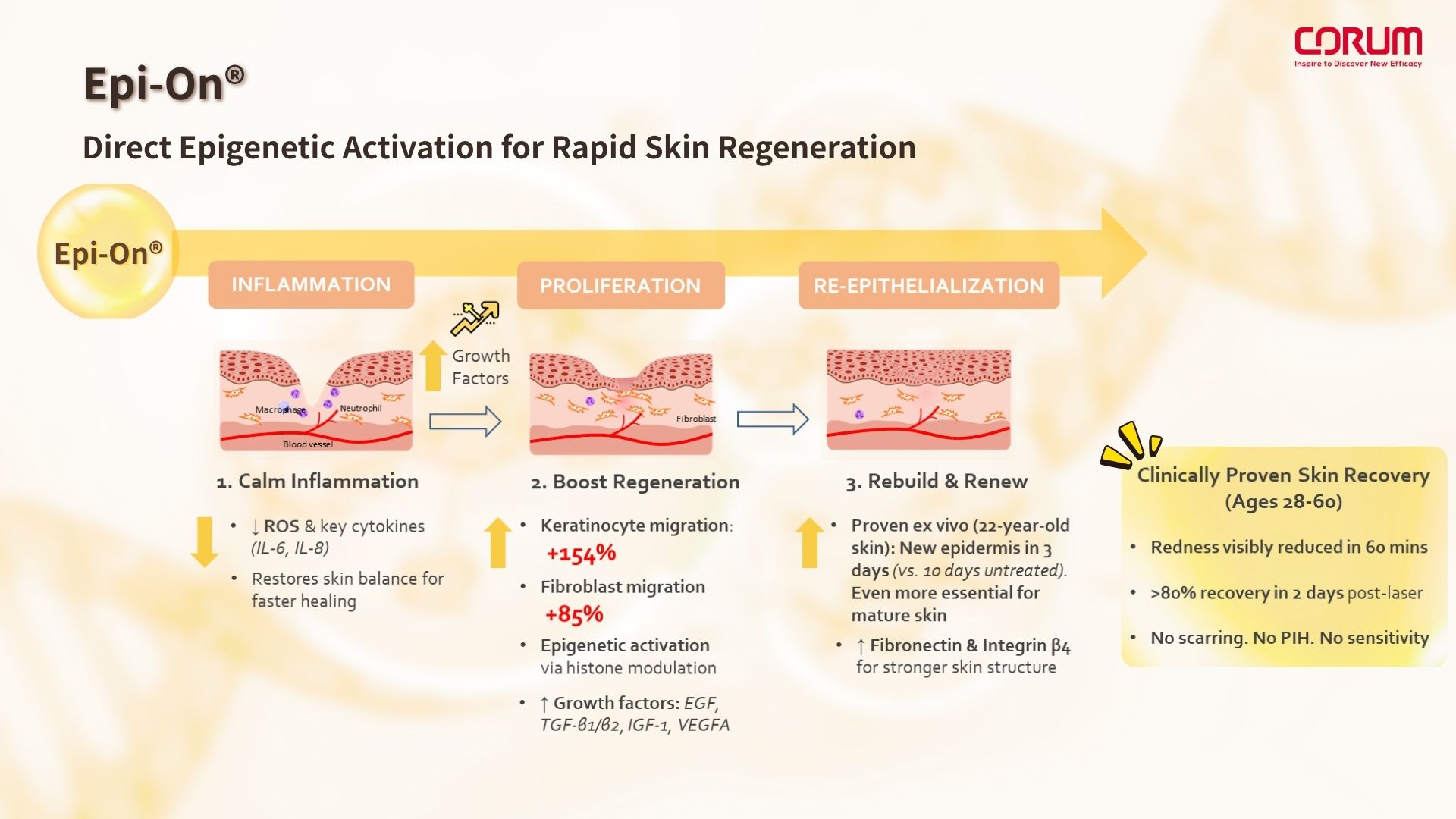
This small-molecule epigenetic modulator offers more immediate, controlled, and cell-aligned regenerative potential. With a molecular weight of just ~272 Da, it fits within the optimal range for transdermal absorption (<500 Da). Epi-On® can effectively penetrate the stratum corneum, allowing for topical delivery that triggers repair signaling quickly and precisely.
This marks the beginning of a non-invasive, daily-applicable approach to skin repair—one that integrates intelligent regeneration and epigenetic modulation, redefining how we restore skin health from the inside out.
Epi-On®: Targeting the Cellular Repair Switch Through Epigenetic Histone Modulation
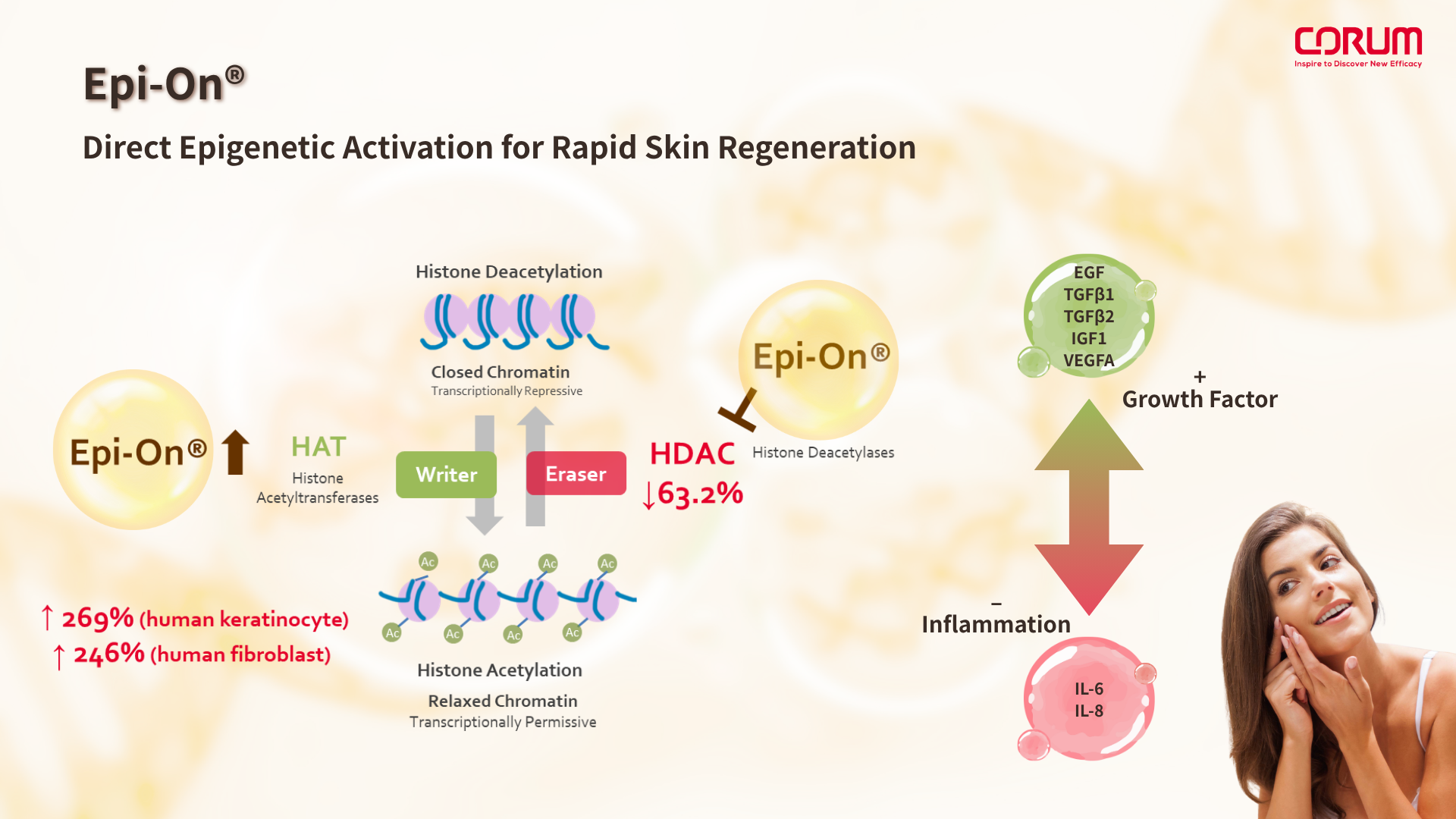
Epi-On® is a next-generation epigenetic regulatory molecule designed to directly enhance histone acetylation, a key modification that plays a central role in regulating anti-inflammatory responses, cellular regeneration, and migration.
This mechanism parallels the downstream effects of PDRN, which relies on metabolic conversion to adenosine within the body to activate similar pathways. However, unlike PDRN, Epi-On® acts as a primary active, requiring no enzymatic conversion, and can directly influence both epigenetic repair and anti-inflammatory signaling. As a result, it offers:
Epi-On® can be seen as an “upstream master redesign” of PDRN’s mechanism of action. By acting directly on histone modification, it enhances repair efficiency while minimizing formulation variability and biological unpredictability, making it a powerful and controllable ingredient in precision skin regeneration strategies.
Rethinking the Exosome Hype: miRNA Profile ≠ Universally Beneficial

Exosomes have recently gained attention as a "next-generation delivery vehicle" in skin repair, praised for their natural origin, strong skin penetration, and their ability to carry a wide range of bioactive signals, including:
However, this multi-signal packaging also brings a level of compositional uncertainty. In particular, the miRNA profile within exosomes can be unpredictable and, without selective screening or targeted engineering, may introduce signaling interference that works against the intended repair objectives.
For example, certain miRNAs are known to counteract skin regeneration and healing pathways. Their unintended presence in a formulation could interfere with or weaken the performance of other carefully selected active ingredients.
Precision Formulation Strategy: Peptides x Epi-On® Outperform Exosome x Random Actives

While traditional exosome models may offer an initial regenerative stimulus, they lack precision. If the exosome cargo does not specifically match the skin’s current condition, it may instead introduce instability. Moreover, exosomes require active cellular uptake (endocytosis) to deliver their contents — meaning their effectiveness relies heavily on the viability and activity of recipient cells. In cases of low cellular activity (e.g., severely aged skin), their efficacy is significantly diminished.
In contrast, a targeted repair approach using signaling peptides combined with epigenetic modulators such as Epi-On® enables purposeful formulation design based on specific biological needs — including cell migration, anti-inflammation, and extracellular matrix remodeling. This strategy offers several key advantages:
For clinical applications, this precision-driven combination is especially well-suited for post-procedure recovery, sensitive skin management, and chronic inflammatory skin conditions requiring high-level accuracy and consistency.
From Repair to Homeostatic Balance: Azeclair® P as the Key to Structured Regeneration and Long-Lasting Restoration

Potassium Azeloyl Diglycinate (Azeclair® P) serves as the core regulator of homeostasis in next-generation skin regeneration systems, playing multiple essential roles. Beyond its well-known oil-controlling and brightening properties, our research has revealed that Azeclair® P also promotes the synthesis of glycosaminoglycan (GAG) precursors—the building blocks of hyaluronic acid—as well as the expression of filaggrin, a key precursor of natural moisturizing factors (NMFs). This strengthens moisture circulation and hydration function within the stratum corneum.
On the lipid level, Azeclair® P helps regulate sebum production, while also enhancing the replenishment and organization of intercellular lipids. This contributes to repairing damaged lipid bilayers, improving the skin barrier’s moisture retention capacity and structural integrity.
In terms of structural proteins, Azeclair® P increases the expression of critical components of the cornified envelope, such as envoplakin and loricrin—both essential for maintaining corneocyte alignment and physical barrier function.
This synergistic mechanism—spanning hydration, lipid restoration, and protein reinforcement—positions Azeclair® P as a vital factor in maintaining a balanced skin microenvironment, and a key player in intelligent regenerative repair.
Conclusion: From Biomimicry to Precision Regeneration — Skin Repair Enters the Era of Intelligent Design

In traditional skin regeneration strategies, PDRN and Sodium DNA once played a key role as initiators. Through nucleic acid metabolism, they generate adenosine, which activates A2A receptors and subsequently triggers the cAMP/PKA signaling pathway. Protein kinase A (PKA) not only directly phosphorylates histones (e.g., H3S10), leading to chromatin relaxation, but also activates transcription factors like CREB, which then recruit coactivators such as CBP/p300 with histone acetyltransferase (HAT) activity. This process enhances histone acetylation, promoting the transcription of genes related to repair and regeneration.
Though PDRN is not a classical epigenetic regulator, its indirect modulation of transcriptional environments helps initiate favorable conditions for cellular regeneration — effectively serving as a preparatory agent that enables more efficient downstream repair.
Meanwhile, exosomes act as intercellular “messengers,” delivering crucial molecules like miRNAs that can initiate repair mechanisms. Although miRNA was first discovered in 1993, it regained significant attention following the 2024 Nobel Prize in Physiology or Medicine. With advancements in exosome isolation and purification techniques (e.g., ultracentrifugation, nanofiltration, tangential flow filtration), scalable production has become feasible. This has led to their adoption in cosmetic and regenerative skincare, positioning exosomes and RNA-based actives as trending ingredients in skin repair.
The popularity of PDRN and exosomes reflects growing recognition of the skin’s intrinsic self-repair capacity. However, to enhance efficacy, precision, and stability, we must move beyond metabolic intermediates and heterogeneous signaling models. The emergence of Epi-On® marks a shift into a new era where epigenetic regulation and precision protein signaling drive regeneration.
The Next Phase of Skin Regeneration: Peptides × Epi-On®
Unlike passive biomimicry, signaling peptides are engineered to replicate key active sequences found in natural hormones or signaling proteins. These short-chain molecules bind to specific receptors or signaling pathways on fibroblasts, directly delivering biological instructions to stimulate collagen and elastin production — initiating true dermal rebuilding and renewal. They serve as core molecules actively participating in structural remodeling.
At the same time, Epi-On® operates at the epigenetic level, modulating gene expression related to healing, proliferation, and regeneration. This dual mechanism enables precise activation of repair pathways across both the epidermis and dermis, achieving structural repair × biological renewal. Future skin repair systems will no longer rely solely on nature-inspired mimics, but will instead harness the power of high-precision molecular design and signal-guided regeneration for faster, more stable, and more targeted outcomes.
Azeclair® P : The Gatekeeper of Skin Homeostasis
Equally critical is Azeclair® P, the homeostatic regulator within this system. Corum Inc. discovered that Azeclair® P promotes GAG (glycosaminoglycan) synthesis and filaggrin expression, reinforcing the circular hydration cycle between the stratum corneum and dermis. It also helps regulate sebum production, inhibits inflammatory mediators (IL-6, IL-8), enhances keratin synthesis, and repairs intercellular lipids and the external skin barrier. As such, Azeclair® P plays a vital role in maintaining a stable skin microenvironment where regeneration and homeostasis coexist.
The Future of Skin Repair: Signal-Driven × Microenvironment-Optimized
From nucleotides to peptides, from mixed-signal delivery to precise epigenetic control, skin science has evolved from passive metabolic repair to active, precision-driven regeneration. It’s no longer about stacking multiple active ingredients, but about designing high-accuracy, system-based regenerative strategies, guided by targeted signaling and microenvironmental optimization. Future repair models will move beyond “repair-only” to embrace a dual approach: regeneration + homeostasis, awakening the skin’s inherent renewal capacity and achieving true, long-term biological youth.
The R&D Mission at Corum: Deliver Only What the Skin Truly Needs
This vision reflects Corum’s unwavering commitment to skin biology. Our focus lies in providing the skin with only what it truly needs — delivering precise signals and molecular tools for environmental rebuilding. Our innovations include:
Together, these tools address three essential dimensions: hydration, lipid barrier integrity, and protein renewal, crafting a long-term regenerative environment for skin homeostasis.
As skin science enters the era of precision and intelligent regeneration, Corum continues to lead as a technology pioneer in both dermatological and cosmetic innovation — with science at the core, and skin health at the heart.
Formulator’s Note: Key Considerations for Active Ingredient Selection
References: